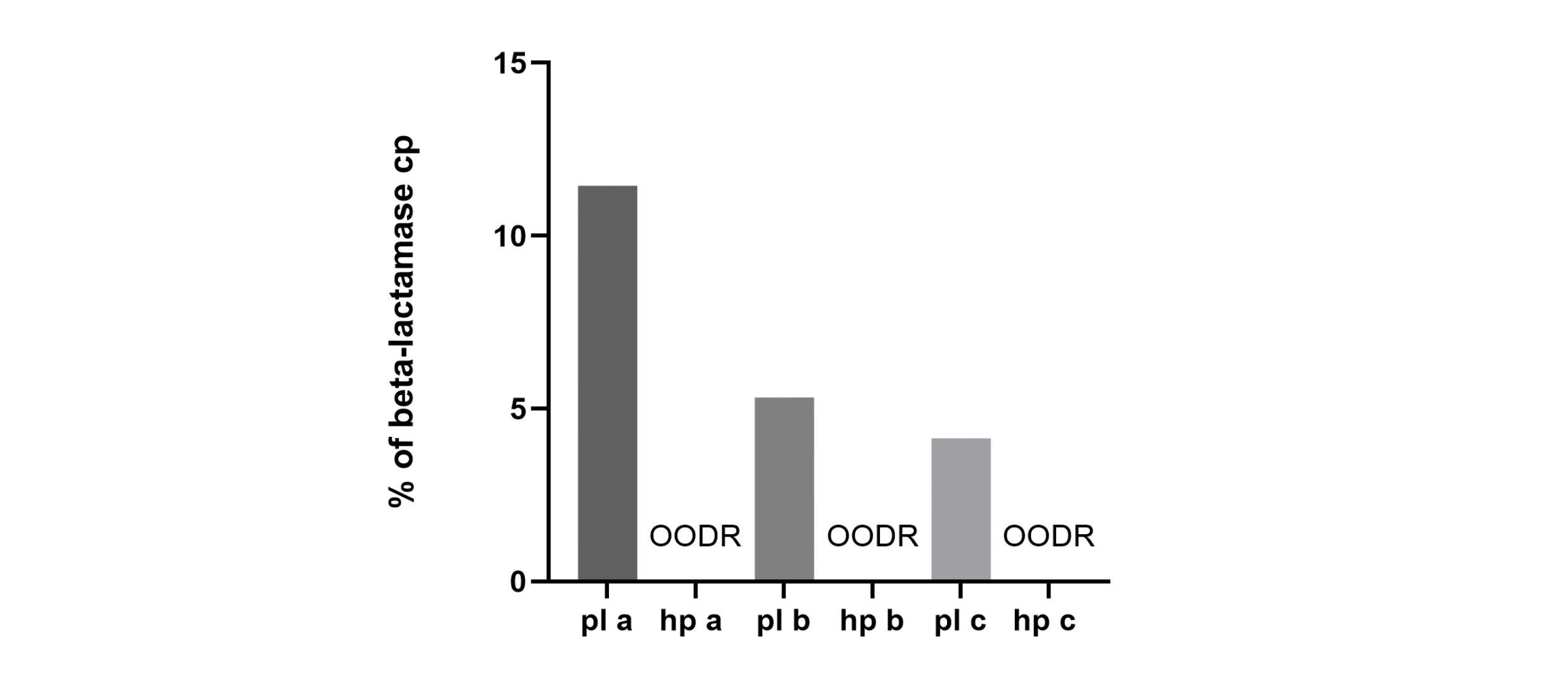Viral Vectors
For cutting-edge pharma and biotech innovators developing next-generation gene therapies using viral vector modalities, the “standard” shouldn’t be a bottleneck. Our hpDNA template is engineered through a unique cell-free manufacturing process to meet the stringent demands of tomorrow's viral vector medicine.
The future of gene therapy is linear
Rethink your template. Revolutionize your results.
The future of high-fidelity DNA templates is synthetic and cell-free.
Our hpDNA technology offers a revolutionary alternative to traditional plasmid DNA (pDNA) by providing a linear, high-fidelity template engineered with structural hairpins and protected 3’ ends. By leveraging a proprietary enzymatic cell-free manufacturing process, we eliminate the biological risks inherent in plasmid backbones, such as host-cell contamination and the inclusion of antibiotic resistance genes. This streamlined approach ensures the highest levels of safety and regulatory compliance, providing a cleaner, more reliable foundation for advanced genetic research and therapeutic development.
Let our DNA experts customize a solution just for you.
hpDNA platform: Purity, safety, and accelerated viral vector development
High-fidelity, linear DNA templates feature unique hairpin structures and protected 3’ and 5’ ends, offering a cleaner, safer, and more efficient alternative to legacy plasmids.
Our unique hpDNA platform improves the core of your viral vector processes:
Unparalleled safety & purity: Our unique cell-free manufacturing process eliminates the plasmid backbone. This means we remove the risk of host-cell contamination and the use of antibiotic resistance genes, ensuring the highest safety and regulatory compliance for your therapeutic product.
Equivalent titres: Achieve equivalent performance as pDNA with ~30% less DNA mass and transfection reagent required
Reduced costs & timelines: By removing the need for complex, time-consuming purification steps required to eliminate contaminants, hpDNA helps you streamline your workflow, significantly reducing costs and accelerating development timelines.
Superior fidelity & performance: As a fully-linearized template with covalently close 3’ and 5’ ends, hpDNA is optimized for your viral vector development, resulting in improved yields and consistent, high-fidelity gene delivery vectors.
The comparative view:
hpDNA vs. pDNA: A head-to-head analysis of next-gen AAV production
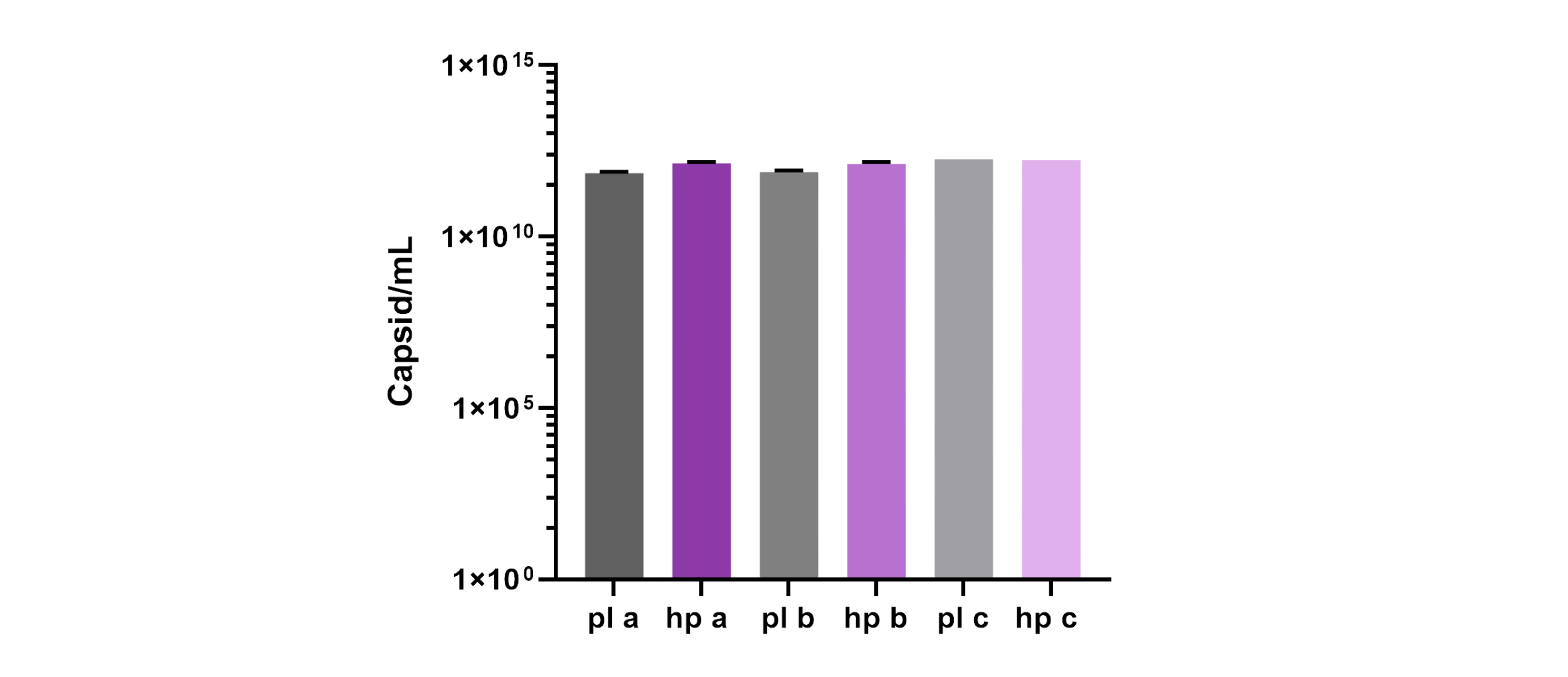
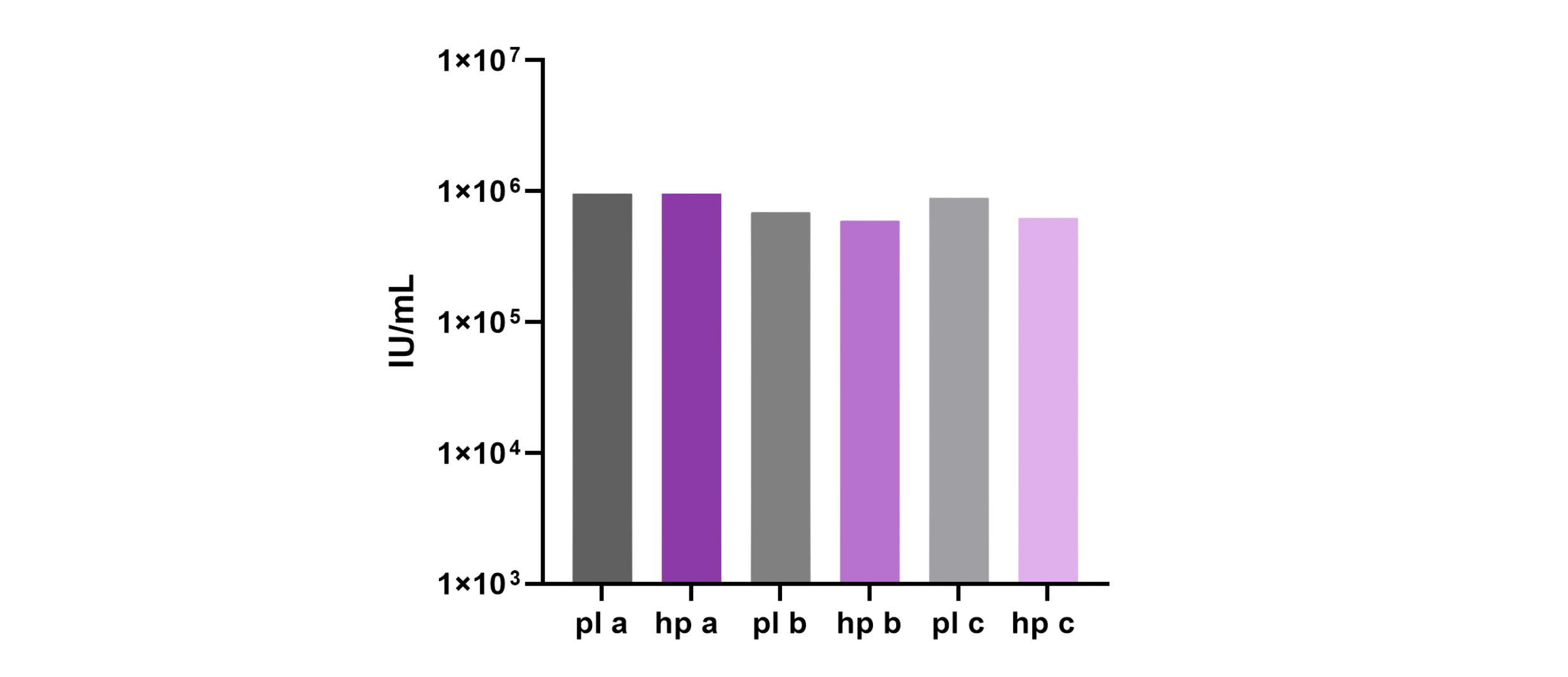
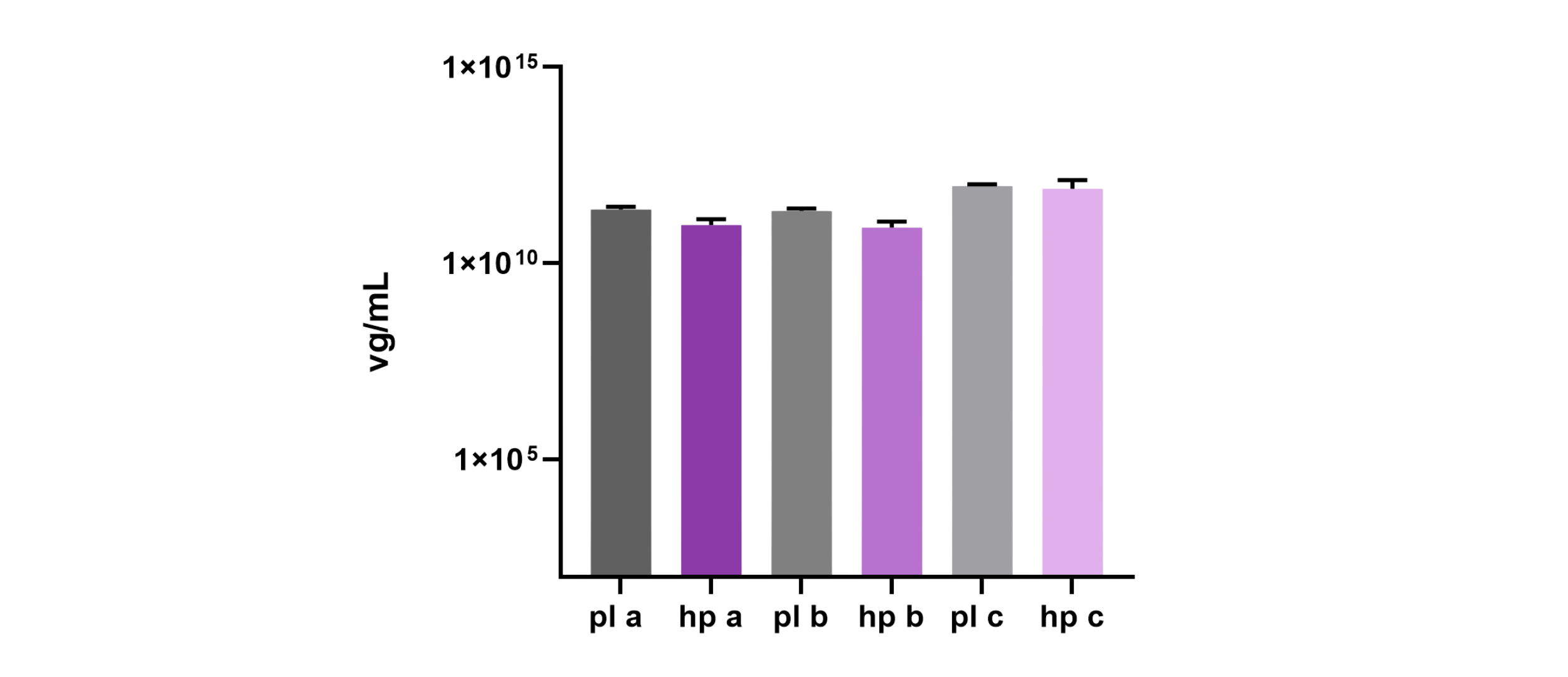
During this head-to-head evaluation of 4basebio’s hpDNA template and traditional pDNA, transfection conditions were optimized for the hpDNA technology, leading to comparable physical and capsid titres compared to pDNA. Data shown below is for rAAV production in multilayer flasks. Infectivity demonstrated by transduction assay, showed comparable transduction of rAAVs produced from hpDNA and plasmid.
This was achieved with a 30% reduction in DNA mass due to the absence of bacterial backbone sequences in hpDNA, which is produced via the amplification of circular, backbone-free templates. PCR of beta-lactamase sequences confirmed the absence of encapsidated bacterial backbone in rAAV produced from hpDNA.
Physical titres
Infectivity (AAV2)
Capsid titres
Backbone detection by qPCR
Learn more about hpDNA technology with our recent blog post
Technology
Find out more about our enzymatic manufacturing process and the benefits of synthetic DNA.
Custom Manufacturing
Discover our RUO, HQ, and GMP-grade synthetic DNA, designed for every stage, from discovery to commercial production