Your DNA partner for every phase
Seamlessly transition from preclinical studies to commercial supply with our cell-free, GMP-aligned synthetic DNA platform
Flexible, high-quality synthetic DNA manufacturing
To meet the growing demands of cell and gene therapy development, we offer a comprehensive suite of synthetic, cell-free DNA manufacturing services designed to support you at every stage, from lead-candidate optimization and preclinical studies through to clinical development and commercial production.
Our custom DNA manufacturing services are tailored to your specific needs. With a focus on quality, speed and efficiency, we provide flexible DNA solutions fit for DNA vaccines, cell and gene therapies as well as gene editing and mRNA therapeutics.
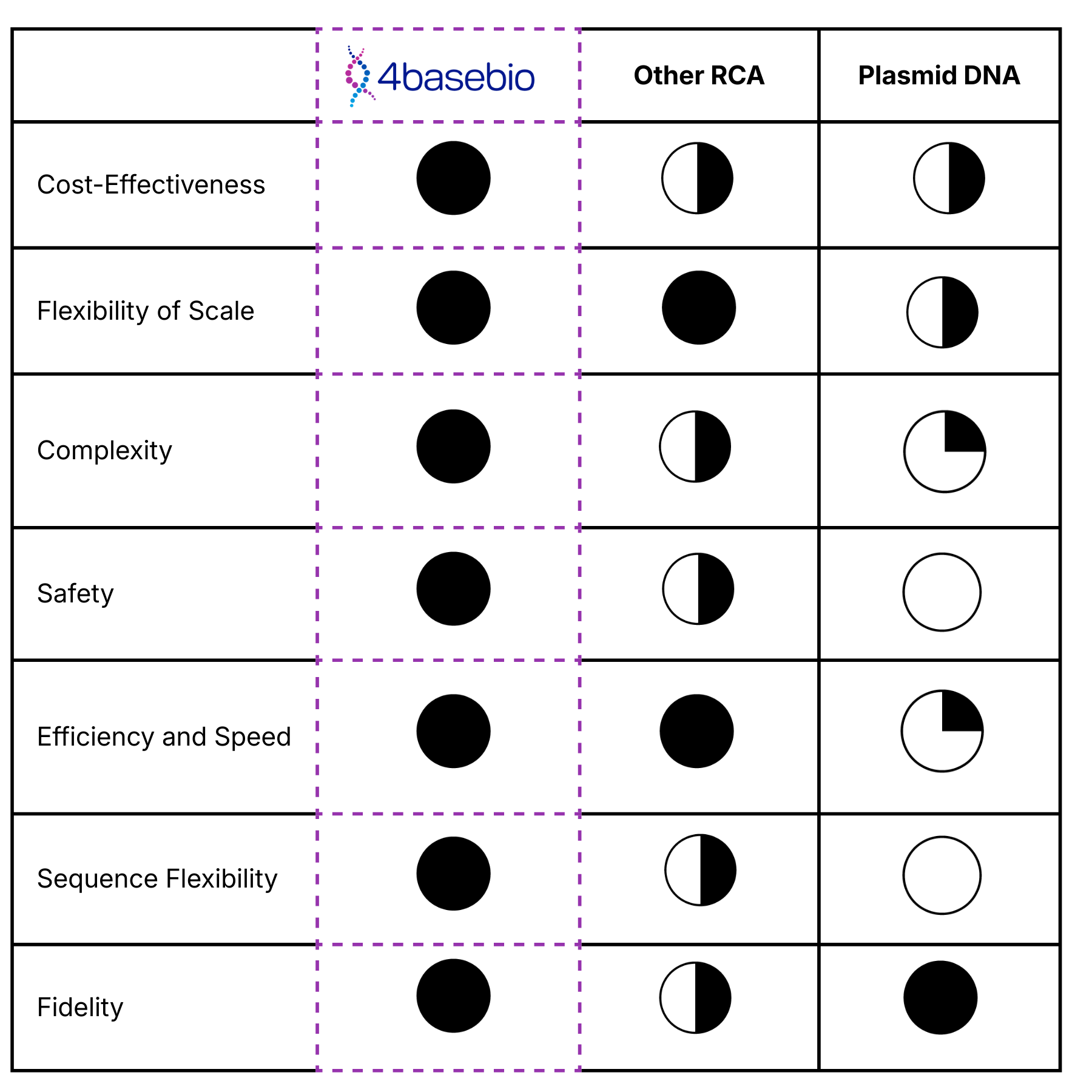
Why make the switch?
Unlike traditional plasmid DNA, our synthetic DNA platform provides a next-generation alternative that accelerates therapeutic development while enhancing safety and efficiency. Produced with our proprietary enzymatic process, our DNA is free from bacterial sequences, antibiotic resistance genes, and endotoxins, eliminating many of the regulatory and process-related challenges associated with plasmid-derived materials.
Ready to make the switch? Request a quote today.
Streamlined DNA production enabled by cell-free DNA
Cell-free synthetic DNA
Traditional plasmid DNA
4basebio’s cell-free DNA technology delivers significantly faster turnaround times than traditional plasmid-based manufacturing. Our streamlined process accelerates the production of high-quality DNA to a matter of weeks rather than months. This speed enables researchers and developers to advance life-changing genetic medicines more efficiently, helping bring innovative therapies to patients who need them most.
Your Partner from pre-clinical to commercial production
4basebio offers three synthetic DNA quality grades, Research, HQ, and GMP, designed to match every stage of development. Built on the same cell-free platform, each grade delivers consistent quality, faster timelines, and scalability from early-stages through to clinical and commercial manufacture.
| Specification | RUO | HQ | GMP Compliant |
|
|---|---|---|---|---|
| Product | Formulation | |||
| DNA characterisation | ||||
| Certification | Certificate of Analysis | |||
| Statement of Origin | ||||
| Quality | Batch manufacturing record | |||
| Qualified testing & production methods | ||||
| QMS controlled | ||||
| Environment | Grade C cleanroom | |||
| Dedicated suite during manufacture | ||||
| Materials | GMP certified raw materials | |||
| Compliance | EU, GMP, Volume 4, Part II |
| Specification | RUO | HQ | GMP Compliant |
|---|---|---|---|
| Product | |||
| Formulation | |||
| DNA characterisation | |||
| Certification | |||
| Certificate of analysis | |||
| Statement of origin | |||
| Quality | |||
| Batch manufacturing record | |||
| Qualified testing & production methods | |||
| QMS controlled | |||
| Environment | |||
| Grade C cleanroom | |||
| Dedicated suite during manufacture | |||
| Materials | |||
| GMP certified raw materials | |||
| Compliance | |||
| EU, GMP, Volume 4, Part II | |||
Key
Research-grade DNA (RUO)
Small batch sizes starting from 1mg
Suitable for initial POC and lead-candidate optimization
GMP-grade DNA (GMP)
Batch sizes from 50mg to 10g
Suitable for late preclinical to commercialization stages
High-quality DNA (HQ)
Batch sizes from 50mg to 10g
Suitable for pre-clinical and toxicology studies
Applications
Find out more about our enzymatic manufacturing process and the benefits of synthetic DNA for your application of interest.
OTS DNA products
Discover our selection of DNA evaluation products, designed to help you test and explore the capabilities of 4basebio’s synthetic, cell-free DNA.