Celebrating 60 Years of AAV: From Discovery to the Future of Gene Therapy
Sixty years ago, the scientific community met a humble but revolutionary virus Adeno-Associated Virus (AAV). Discovered in 1965 as a contaminant in adenovirus preparations, AAV has since transformed from an academic curiosity into one of the most powerful vectors in gene therapy. This year, we celebrate not only its anniversary but it’s remarkable journey through innovation.
The rise of AAV in gene therapy
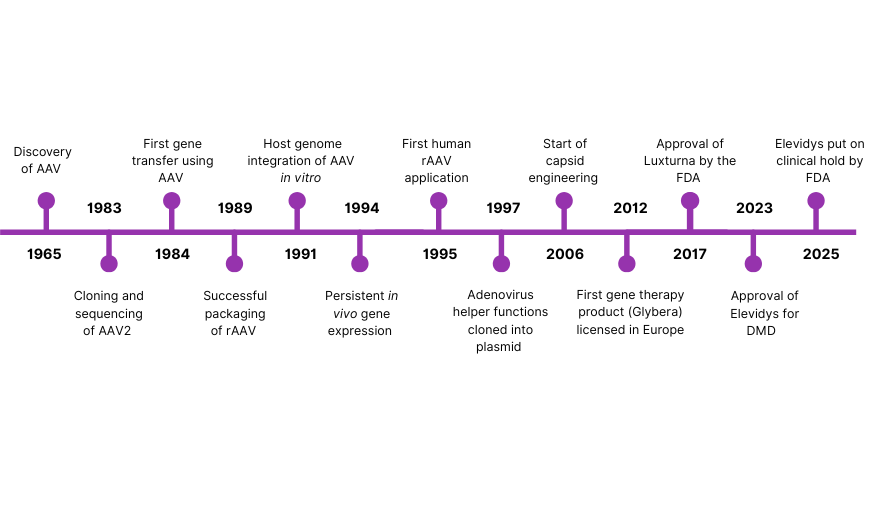
Discovered by Bob Atchison, M. David Hoggan, and Wallace Rowe in 1965, AAV was characterized as a virus that replicated poorly in human cells in the absence of a helper virus (1). As molecular biology advanced, scientists began to recognize AAV’s potential as a gene delivery tool. Its ability to infect both dividing and non-dividing cells, and its non-pathogenic nature, made it an attractive candidate for gene therapy. Early experiments demonstrated that AAV could integrate into host genomes and express foreign genes with high fidelity.
Figure 1: Major Historical milestones in AAV research and development from the discovery of AAV in 1965 up until 2025
In a groundbreaking moment, 1995 saw the first recombinant AAV (rAAV) vectors administered to humans in a clinical trial targeting cystic fibrosis (2). Though the results were modest, the study was a major milestone, proving the concept that AAV could be safely used in human gene therapy. A pivotal advancement came in 1997, when the adenoviral helper functions were cloned into a plasmid, replacing the need for live adenovirus during AAV production (3). This shift to a plasmid-based triple transfection system dramatically improved the safety and scalability of AAV manufacturing, making it more suitable for clinical and commercial applications.
Throughout the early 2000s, gene therapy research using AAV vectors accelerated. Different AAV serotypes were isolated and characterized, expanding the range of tissues that could be targeted (4). Clinical trials showed promise for diseases such as hemophilia, congenital blindness, and muscular dystrophies, reinforcing AAV’s reputation as a leading vector.
A major regulatory breakthrough occurred in 2012 with the approval of Glybera, the world’s first licensed gene therapy using an AAV vector (5). Approved in the European Union, Glybera treated lipoprotein lipase deficiency (LPLD), a rare metabolic disorder. Although later withdrawn for commercial reasons, its approval was historic, it validated AAV as a clinically viable gene delivery tool.
With over 343 AAV clinical trials, rAAV is now an established vector in gene therapy. This can be observed based on the number of new clinical trials which utilize AAVs (Figure 2 below) (1). However, there are still many unknowns about the virus biology, and commercial-scale manufacturing of the recombinant remains challenging. Moreover, the lack of standardization for the full characterization of the final virus batches is posing safety concerns. This issue was highlighted with the recent deaths in the Sarepta clinical trials for limb girdle muscular dystrophy, placing it at the forefront of development and regulatory considerations (6).
Figure 2: Number of gene therapy trials that utilize AAV, since 1995 up until 2024. Reference: (Suarez-Amaran et al., 2025)
Emerging challenges of plasmid DNA
As AAV manufacturing continues to expand to meet clinical and commercial demand, the challenges associated with plasmid DNA are becoming increasingly apparent. Plasmid DNA was revolutionary when it first enabled large-scale AAV production in the late 1990s, but its limitations are now a major constraint. Production relies on bacterial fermentation, a process that is time-consuming, costly, and prone to variability. Consistency across batches is difficult to achieve, as yields and purity often vary, and critical sequences such as inverted terminal repeats (ITRs) are vulnerable to recombination in bacterial systems. In addition, plasmid DNA carries unavoidable risks of endotoxin, bioburden, and genomic DNA contaminants, all of which require extensive purification and rigorous testing.
A New Chapter: Synthetic DNA and the Future of AAV Manufacturing
The emergence of synthetic DNA is opening a new chapter for AAV manufacturing. Produced enzymatically in a cell-free system, synthetic DNA eliminates the need for bacterial fermentation and the safety risks that come with it. 4basebio’s synthetic DNA platform is designed to address many of the industry’s current challenges. It enables faster production of GMP-grade batches, supporting accelerated development timelines. By removing the bacterial backbone prior to DNA amplification, the cell-free manufacturing process delivers safer starting material for AAV production. This step enhances the overall safety profile by reducing the risk of aberrantly incorporating bacterial backbone sequences into the final AAV therapy.
Inverted terminal repeats (ITRs) are critical elements of the AAV genome, serving as the origin of replication and enabling replication, packaging, and long-term vector persistence. However, their structural complexity makes them prone to replication errors and truncations during plasmid propagation in bacteria, which can lead to decreased virus yields. Recent studies have shown that around 40% of AAV transfer plasmids had at least one ITR deviated from the expected sequence, showing the prevalent issue of ITR instability in pDNA. (7) By contrast, 4basebio’s enzymatic synthetic DNA process is fully compatible with ITRs, allowing reliable propagation of these sequences.
Importantly, synthetic DNA also improves the cost-effectiveness of AAV production. Its optimized linear constructs require less DNA and fewer transfection reagents to achieve equivalent titers compared with plasmid DNA, directly reducing material requirements. Moreover, because synthetic DNA production does not depend on master cell banking, it avoids a significant cost driver and operational bottleneck of plasmid manufacturing. Together, these advantages make synthetic DNA an efficient, consistent, and economically viable foundation for the next generation of AAV therapies.
The next chapter for AAV
In the six decades since its discovery, AAV has progressed to a well-established platform for delivering gene therapies. This has been driven by advances in molecular biology, vector design, and manufacturing, particularly the development of plasmid DNA-based production systems.
Looking ahead, the adoption of synthetic DNA and other next-generation manufacturing methods is expected to improve both efficiency and safety, supporting broader clinical use. Continued refinement of AAV technologies will be key to meeting the growing demand for reliable, effective genetic medicines.
References:
Suarez-Amaran, L., Song, L., Tretiakova, A. P., Mikhail, S. A., & Samulski, R. J. (2025). AAV vector development, back to the future. Molecular Therapy, 33(5), 1903–1936. https://doi.org/10.1016/j.ymthe.2025.03.064
Wang, J.H., Gessler, D. J., Zhan, W., Gallagher, T. L., & Gao, G. (2024). Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduction and Targeted Therapy, 9(1). https://doi.org/10.1038/s41392-024-01780-w
Li, J., Samulski, R. J., & Xiao, X. (1997). Role for highly regulated rep gene expression in adeno-associated virus vector production. Journal of Virology, 71(7), 5236–5243. https://doi.org/10.1128/jvi.71.7.5236-5243.1997
Hastie, E., & Samulski, R. J. (2015). Adeno-associated virus at 50: A golden anniversary of Discovery, research, and gene therapy success—A personal perspective. Human Gene Therapy, 26(5), 257–265. https://doi.org/10.1089/hum.2015.025
Flotte, T. R. (2013). Birth of a new therapeutic platform: 47 years of adeno-associated virus biology from virus discovery to licensed gene therapy. Molecular Therapy, 21(11), 1976–1981. https://doi.org/10.1038/mt.2013.226
Bilodeau, K. (2025, March 31). A recent gene therapy death shines a light on Aav Safety. PharmaVoice. https://www.pharmavoice.com/news/sarepta-elevidys-gene-therapy-duchenne-death-aav-safety/743824/
Bai, X., Hong, J. F., Yu, S., Hu, D. Y., Chen, A. Y., Rich, C. A., Shi, S. J., Xu, S. Y., Croucher, D. M., Müssar, K. J., Meng, D. W., Chen, J. L., & Lahn, B. T. (2025). Prevalence of errors in lab-made plasmids across the Globe. Nucleic Acids Research, 53(14). https://doi.org/10.1101/2024.06.17.596931
Brimble, M. A., Winston, S. M., & Davidoff, A. M. (2023). Stowaways in the cargo: Contaminating nucleic acids in Raav preparations for gene therapy. Molecular Therapy, 31(10), 2826–2838. https://doi.org/10.1016/j.ymthe.2023.07.025
Wang, D., Tai, P. W., & Gao, G. (2019). Adeno-associated virus vector as a platform for gene therapy delivery. Nature Reviews Drug Discovery, 18(5), 358–378. https://doi.org/10.1038/s41573-019-0012-9